Efficacy of Tranexamic Acid in Reducing Blood Loss in Cesarean Section: A Comparative Study.
DOI:
https://doi.org/10.22502/jlmc.v7i2.292Keywords:
Blood loss, Cesarean section, Tranexamic acidAbstract
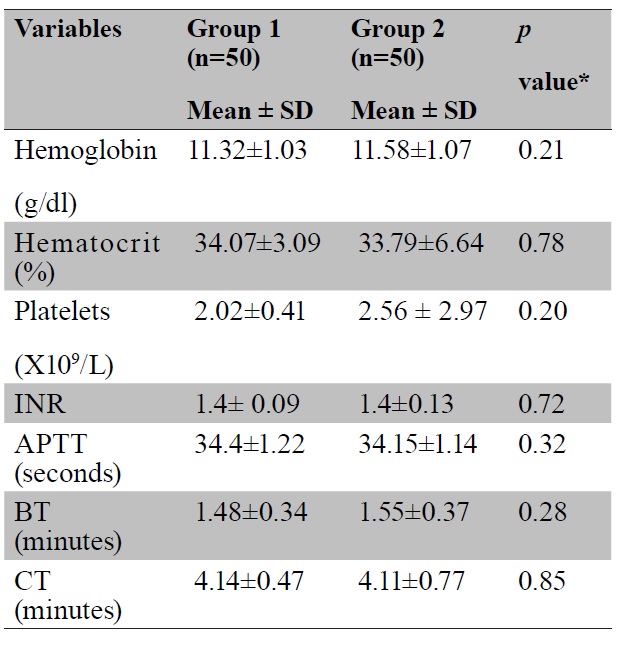
Introduction: Obstetric hemorrhage is one of the major causes of maternal morbidity and mortality. Blood loss during cesarean section is almost twice than that in vaginal delivery. The aim of this study was to evaluate the efficacy of tranexamic acid to reduce blood loss in cesarean section and its side effects. Methods: A comparative study was done in 100 women undergoing cesarean section between December 2015 to January 2017. The study group of 50 women received one gram intravenous tranexamic acid and the control group of 50 women did not receive tranexamic acid. Primary outcome measure was blood loss during cesarean section. Secondary outcome measures were drop in post-operative hemoglobin and hematocrit, change in pulse rate and blood pressure, need of additional uterotonics, auxiliary procedures to stop bleeding, blood transfusion rate and maternal and neonatal side effects of the drug. Results: Mean intraoperative blood loss in the study group was 443.62± 86.73ml; and in control group, 667.40±131.01ml (p<0.001). Mean postoperative drop in hemoglobin (g/dl) in the two groups were 0.82±0.27 and 1.86±0.64 respectively (p<0.001). Mean postoperative drop in hematocrit in the two groups were 2.60±0.91 and 5.49±1.97 respectively (p<0.001). Fourteen patients in the control group required additional uterotonics while none in the study group (p<0.001). There was no significant difference in the transfusion requirement (p=0.079). None of the mothers and the newborns had major side effects of drug. Conclusion: Tranexamic acid is a safe and effective drug to reduce blood loss in cesarean section.
Downloads
References
Inter-Agency and Expert Group on Millennium Development Goals Indicators. The millennium development goals report 2015. Department of Economic and Social Affairs of the United Nations Secretariat. 2015. Available from: https://bit.ly/37KmtJt
WHO. The World Health Report: 2005: Make Every Mother and Child Count. World Health Organization. 2005. Available from: https:// bit.ly/2OqXwLn
Wellington K, Wagstaff AJ. Tranexamic acid: review of its use in management of menorrhagia. Drugs. 2003;63(13):1417-33. PMID: 12825966 DOI: 10.2165/00003495- 200363130-00008
Roberts I. Tranexamic acid in trauma: how should we use it? J Thromb Haemost. 2015;13(Suppl 1):S195–9. PMID: 26149023 DOI: https://doi.org/10.1111/jth.12878
CRASH-2 trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23– 32. PMID: 20554319 DOI: 10.1016/S0140- 6736(10)60835-5
Walker G, Association of British Pharmaceutical Industry. ABPI Compendium of data sheets and summaries of product characteristics, 1998-99: with the code of practice for the pharmaceutical industry. London : Datapharm Publication; 1998.
Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet. 2006;368(9542):P1189–1200. DOI:10.1016/S0140-6736(06)69380-X
Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e33. PMID: 25103301 DOI: 10.1016/S2214-109X(14)70227-X
Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after cesarean section: a multi-center, randomized trial. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):154–7. PMID: 14746950. DOI: https://doi.org/10.1016/ s0301-2115(03)00287-2
Bhavana G, Abhishek MV, Mittal S. Efficacy of prophylactic tranexamic acid in reducing blood loss during and after cesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 2016;5(6):2011-6. DOI: 10.18203/2320-1770. ijrcog20161708
Yehia AH, Koleib MH, Abdelazim IA, Atik A et al. Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial. Asian Pacific Journal of Reproduction. 2014;3(1):53- 6. DOI: 10.1016/S2305-0500(14)60002-6
Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. The Journal of Obstetrics and Gynecology of India. 2007;57(3):227–30. Available from: https:// bit.ly/37LdDLJ
WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. DOI: 10.1016/S0140- 6736(17)30638-4
Bekassy Z, Astedt B. Treatment with the fibrinolytic inhibitor tranexamic acid-risk for thrombosis? Acta Obstetricia et Gynecologica Scandinavica. 1990;69(4):353–4. DOI: 10.3109/00016349009036161
Gungorduk K, Asıcıoğlu O, Yıldırım G, Ark C, Tekirdağ Aİ, Besımoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. Am J Perinatol. 2013;30(5):407–13. PMID: 23023559 DOI: 10.1055/s-0032-1326986
Svanberg L, Astedt B, Nilsson IM. Abruptio placentae-treatment with the fibrinolytic inhibitor tranexamic acid. Acta Obstericiat et Gynecologica Scandinavica. 1980;59(2):127– 30. DOI: 10.3109/00016348009154628
Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. International Journal of Gynaecology & Obstetrics. 2011;115(3):224–26. DOI: https://doi.org/10.1016/j.ijgo.2011.07.015
Downloads
Published
Issue
Section
License
- The Journal of Lumbini Medical College (JLMC) publishes open access articles under the terms of the Creative Commons Attribution (CC BY) License which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
- JLMC requires an exclusive licence to publish the article first in its journal in print and online.
- The corresponding author should read and agree to the following statement before submission of the manuscript for publication,
- License agreement
- In submitting an article to Journal of Lumbini Medical College (JLMC) I certify that:
- I am authorized by my co-authors to enter into these arrangements.
- I warrant, on behalf of myself and my co-authors, that:
- the article is original, has not been formally published in any other peer-reviewed journal, is not under consideration by any other journal and does not infringe any existing copyright or any other third party rights;
- I am/we are the sole author(s) of the article and have full authority to enter into this agreement and in granting rights to JLMC are not in breach of any other obligation;
- the article contains nothing that is unlawful, libellous, or which would, if published, constitute a breach of contract or of confidence or of commitment given to secrecy;
- I/we have taken due care to ensure the integrity of the article. To my/our - and currently accepted scientific - knowledge all statements contained in it purporting to be facts are true and any formula or instruction contained in the article will not, if followed accurately, cause any injury, illness or damage to the user.
- I, and all co-authors, agree that the article, if editorially accepted for publication, shall be licensed under the Creative Commons Attribution License 4.0. If the law requires that the article be published in the public domain, I/we will notify JLMC at the time of submission, and in such cases the article shall be released under the Creative Commons 1.0 Public Domain Dedication waiver. For the avoidance of doubt it is stated that sections 1 and 2 of this license agreement shall apply and prevail regardless of whether the article is published under Creative Commons Attribution License 4.0 or the Creative Commons 1.0 Public Domain Dedication waiver.
- I, and all co-authors, agree that, if the article is editorially accepted for publication in JLMC, data included in the article shall be made available under the Creative Commons 1.0 Public Domain Dedication waiver, unless otherwise stated. For the avoidance of doubt it is stated that sections 1, 2, and 3 of this license agreement shall apply and prevail.
Please visit Creative Commons web page for details of the terms.












